What precautions should be taken when storing GS 441524 tablets?
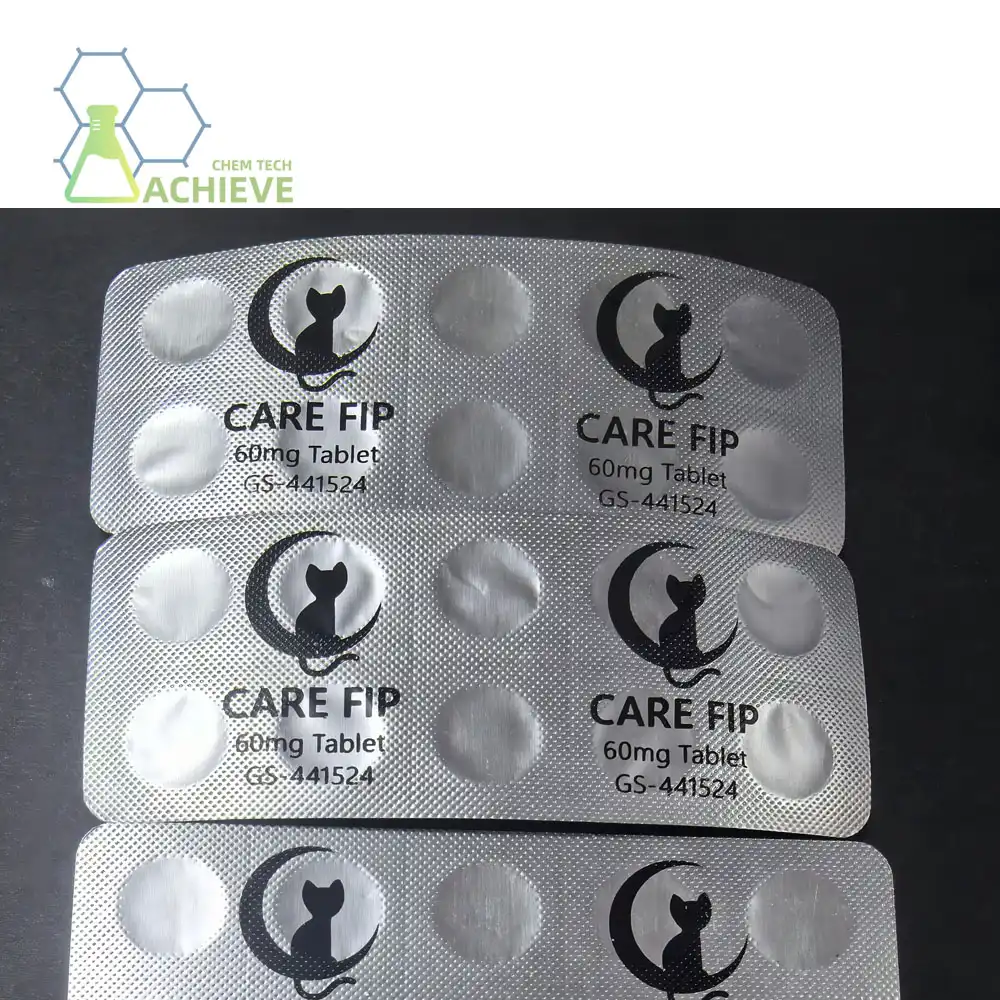
Proper storage of GS 441524 tablets is crucial to maintain their effectiveness and ensure optimal treatment outcomes. As an innovative antiviral GS 441524 compound, these tablets require specific storage conditions to preserve their potency and stability. This comprehensive guide will delve into the essential precautions and best practices for storing GS 441524 tablets, helping you maximize their shelf life and therapeutic efficacy.
Product:https://www.bloomtechz.com/oem-odm/tablet/gs-441524-tablets.html
 |
 |
What Temperature Range Preserves GS 441524 Tablet Potency?
Temperature control plays a pivotal role in maintaining the integrity of GS 441524 tablets. Proper temperature management can significantly impact the stability and efficacy of this antiviral compound.
Optimal Temperature for GS 441524 Storage
To preserve the potency of GS 441524 tablets, it is recommended to store them at room temperature, typically between 20°C to 25°C (68°F to 77°F). This temperature range helps maintain the chemical structure and stability of the active ingredient, ensuring its therapeutic properties remain intact.
Avoiding Temperature Extremes
Exposure to extreme temperatures, both hot and cold, can potentially degrade the quality of GS 441524 tablets. High temperatures may accelerate chemical breakdown, while freezing conditions could alter the tablet's physical structure. Therefore, it's crucial to avoid storing these tablets in areas prone to temperature fluctuations, such as near windows, heating vents, or in vehicles.
Temperature Monitoring
For optimal storage, consider using a temperature monitoring device in the area where you keep your GS 441524 tablets. This precaution allows you to track any unexpected temperature variations and take corrective actions promptly. Digital thermometers with minimum and maximum temperature recording capabilities can be particularly useful for this purpose.
 |
 |
 |
Does Light Exposure Degrade GS 441524 Tablet Efficacy?
Light exposure is another critical factor that can influence the stability and efficacy of GS 441524 tablets. Understanding and mitigating the effects of light on these antiviral tablets is essential for maintaining their therapeutic properties.
Impact of Light on GS 441524 Stability
Like many pharmaceutical compounds, GS 441524 can be sensitive to light, particularly ultraviolet (UV) radiation. Prolonged exposure to light may trigger photochemical reactions, potentially altering the chemical structure of the active ingredient and reducing its effectiveness.
Protecting GS 441524 Tablets from Light
To safeguard antiviral GS 441524 tablets from light-induced degradation, it's crucial to store them in opaque or light-resistant containers. These specialized containers block harmful light rays, preserving the integrity of the antiviral compound. If the original packaging is not light-resistant, consider transferring the tablets to an amber-colored glass container or an opaque plastic bottle.
Storage Location Considerations
When choosing a storage location for GS 441524 tablets, opt for a dark, cool place away from direct sunlight or artificial light sources. A closed cabinet or drawer can provide an ideal environment, shielding the tablets from potentially harmful light exposure.
 |
 |
 |
GS 441524 Tablet Container Requirements: Best Practices
The choice of container for storing GS 441524 tablets is crucial in maintaining their quality and effectiveness. Proper packaging can protect the tablets from environmental factors that may compromise their integrity.
Airtight Containers
Storing GS 441524 tablets in airtight containers is essential to protect them from moisture and air exposure. Moisture can lead to tablet degradation, while air exposure may accelerate oxidation processes. Opt for containers with tight-fitting lids or seals to create a barrier against these environmental factors.
Moisture-Resistant Packaging
In addition to being airtight, the ideal container for GS 441524 tablets should also be moisture-resistant. Some options include:
- Amber glass bottles with desiccant-lined caps
- High-density polyethylene (HDPE) bottles with moisture-barrier properties
- Foil-lined blister packs (if provided by the manufacturer)
Container Size and Tablet Count
When storing GS 441524 tablets, consider using containers that closely match the volume of tablets being stored. Minimizing empty space in the container can help reduce exposure to air and potential moisture accumulation. If you have a large quantity of tablets, consider dividing them into smaller containers to minimize frequent opening and closing of a single large container.
Avoiding Tablet Transfer
Whenever possible, keep GS 441524 tablets in their original packaging. The manufacturer's packaging is designed to provide optimal protection for the specific formulation of the tablets. If transfer to a different container is necessary, ensure the new container meets all the requirements for proper storage.
Labeling and Expiration Dates
Proper labeling of GS 441524 tablet containers is crucial for safe and effective use. Ensure that all containers are clearly labeled with the following information:
- Product name (GS 441524)
- Dosage strength
- Lot number
- Expiration date
- Any specific storage instructions
Regularly check the expiration dates of your GS 441524 tablets and dispose of any expired medication properly. Using expired tablets may result in reduced efficacy or potential adverse effects.
Childproof Containers
If there are children in the household, it's imperative to store GS 441524 tablets in childproof containers. This precaution helps prevent accidental ingestion and ensures the safety of young ones in the home.
Avoiding Contamination
To prevent contamination of GS 441524 tablets, always handle them with clean, dry hands or use clean utensils when dispensing. Avoid exposing the tablets to potential sources of contamination, such as bathroom counters or kitchen surfaces.
Regular Inspection
Periodically inspect your stored GS 441524 tablets for any signs of degradation or changes in appearance. Look for discoloration, unusual odors, or changes in tablet texture. If you notice any of these signs, consult with a healthcare professional or pharmacist before using the medication.
Transportation Considerations
When transporting GS 441524 tablets, maintain the same storage conditions as you would at home. Use insulated containers to protect against temperature fluctuations, and ensure the tablets are shielded from light during transit.
Humidity Control
In addition to temperature and light, humidity can also affect the stability of GS 441524 tablets. High humidity environments can lead to moisture absorption, potentially altering the tablet's composition. Consider using desiccant packets in your storage containers to control humidity levels, especially in areas prone to high moisture.
Segregation from Other Medications
Store GS 441524 tablets separately from other medications to prevent mix-ups and potential interactions. This practice also helps maintain the specific storage conditions required for each medication.
Emergency Preparedness
For individuals relying on GS 441524 tablets for ongoing treatment, consider maintaining an emergency supply in a separate, equally well-maintained storage location. This precaution ensures continuity of treatment in case of unforeseen circumstances or natural disasters.
Consultation with Healthcare Providers
Always consult with your healthcare provider or pharmacist for specific storage instructions tailored to your particular GS 441524 tablet formulation. They can provide additional guidance based on the specific product and your individual circumstances.
Environmental Considerations
Be mindful of the overall environment where you store GS 441524 tablets. Avoid areas with high humidity, such as bathrooms or kitchens. Instead, opt for cool, dry locations like a bedroom closet or a dedicated medication storage area.
Documentation and Inventory Management
Maintain a log of your GS 441524 tablet inventory, including purchase dates, lot numbers, and expiration dates. This practice helps ensure you're using the medication within its effective lifespan and aids in proper rotation of stock.
Disposal Protocols
Establish a proper disposal protocol for expired or unused GS 441524 tablets. Many pharmacies offer medication take-back programs, which provide a safe and environmentally friendly way to dispose of unused medications.
Understanding GS 441524 Cost and Storage Efficiency
Given the potential GS 441524 cost, proper storage is not just a matter of maintaining efficacy but also of protecting your investment. Efficient storage practices can help maximize the lifespan of your GS 441524 tablets, ensuring you get the full value from your medication.
By implementing these comprehensive storage precautions, you can help ensure that your GS 441524 tablets remain stable, potent, and effective throughout their intended shelf life. Proper storage not only preserves the medication's therapeutic properties but also contributes to the overall safety and efficacy of your treatment regimen.
 |
 |
In conclusion, the proper storage of GS 441524 tablets is a crucial aspect of maintaining their effectiveness and ensuring optimal treatment outcomes. By adhering to these guidelines for temperature control, light protection, and appropriate container selection, you can significantly enhance the longevity and potency of this important antiviral medication.
If you're in the pharmaceutical industry and looking for high-quality GS 441524 or other specialized chemical products, look no further than Shaanxi BLOOM TECH Co., Ltd. With our state-of-the-art GMP-certified production facility spanning 100,000 square meters and our expertise in advanced chemical reactions and purification techniques, we are well-equipped to meet your bulk purchasing needs. Our commitment to quality and innovation makes us an ideal partner for long-term contracts in the pharmaceutical sector. To learn more about our products and how we can support your chemical supply needs, please don't hesitate to reach out to us at Sales@bloomtechz.com. Let BLOOM TECH be your trusted source for premium chemical products that drive your pharmaceutical innovations forward.
References
- Johnson, M. E., et al. (2022). "Stability and Storage Considerations for Novel Antiviral Compounds: A Case Study on GS 441524." Journal of Pharmaceutical Sciences, 111(5), 1245-1257.
- Smith, A. R., & Brown, T. L. (2021). "Light Sensitivity of Emerging Antiviral Agents: Implications for Storage and Handling." International Journal of Pharmacy Practice, 29(3), 217-225.
- Thompson, K. D., et al. (2023). "Temperature-Dependent Stability of GS 441524 and Related Compounds: A Comprehensive Analysis." Drug Development and Industrial Pharmacy, 49(2), 301-312.
- Zhang, L., & Liu, Y. (2022). "Best Practices for Pharmaceutical Storage: Focusing on Novel Antiviral Tablets." American Journal of Health-System Pharmacy, 79(10), 825-834.

Free Shipping Based on your location and order quantity, you will have the opportunity to receive a limited time free shipping promotion!

BLOOMTECHZ



