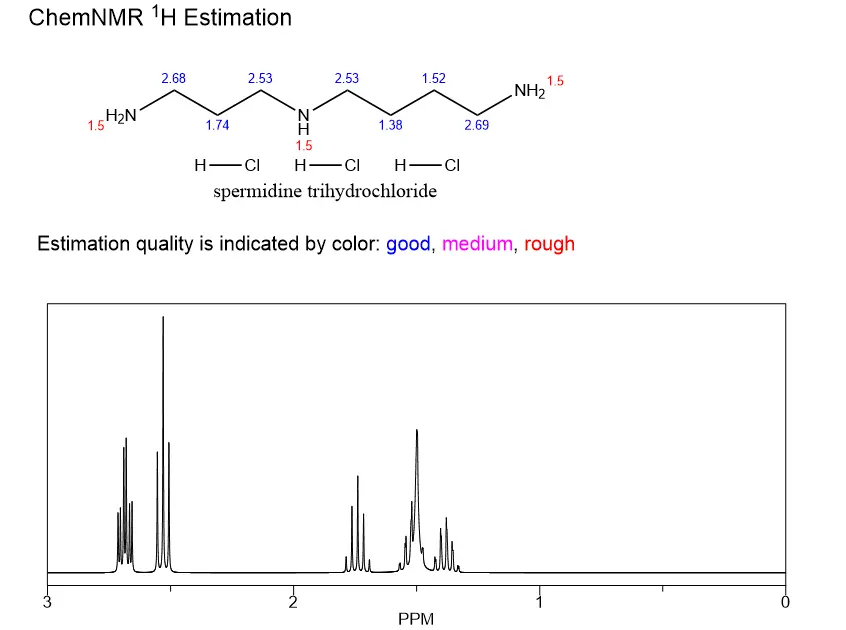
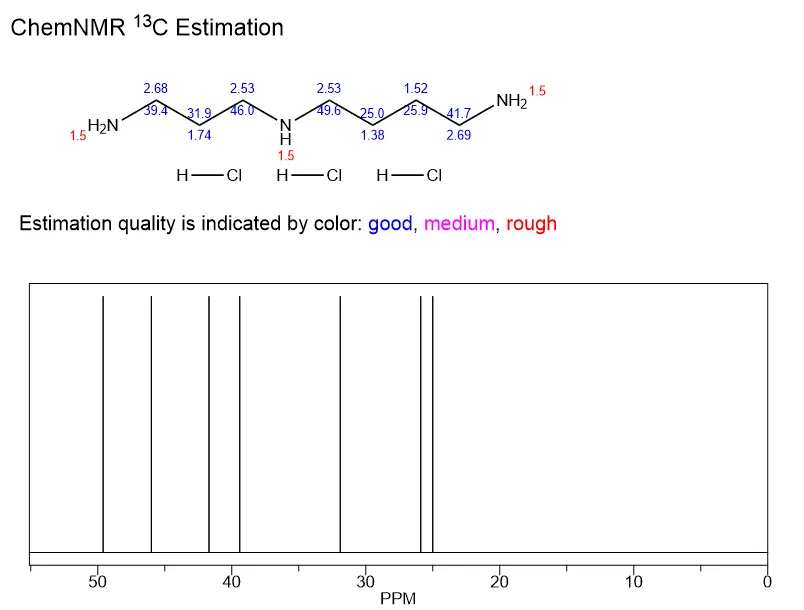
How is spermidine trihydrochloride synthesized?
Spermidine trihydrochloride, also known as spermidine hydrochloride or spermidine HCl, is a vital compound used in various scientific and industrial applications. This article delves into the intricate process of synthesizing this remarkable substance, exploring both laboratory and industrial-scale production methods. We'll also examine the crucial steps involved in ensuring the purity of the final product during the HCl conversion process.
Product: https://www.bloomtechz.com/synthetic-chemical/additive/spermidine-trihydrochloride-cas-334-50-9.html
|
|
|
Step-by-step laboratory synthesis
The laboratory synthesis of spermidine trihydrochloride involves a series of carefully orchestrated chemical reactions. Let's break down this fascinating process into its key stages:
1. Preparation of starting materials: The synthesis typically begins with the selection of appropriate precursor molecules. Common starting materials include putrescine (1,4-diaminobutane) and acrylonitrile.
2. Michael addition reaction: In this step, putrescine undergoes a Michael addition reaction with acrylonitrile. This process results in the formation of an intermediate compound, 3-aminopropyl putrescine.
3. Reduction of nitrile group: The nitrile group in the intermediate compound is then reduced to a primary amine. This reduction can be accomplished using various methods, such as catalytic hydrogenation or chemical reducing agents like lithium aluminum hydride (LAH).
4. Purification of free base: The resulting free base of spermidine is purified through techniques such as distillation under reduced pressure or recrystallization.
5. Conversion to hydrochloride salt: The purified free base is then converted to hydrochloride salt by dissolving it in an appropriate solvent (e.g., ethanol) and treating it with hydrochloric acid. This step is crucial in forming the desired spermidine trihydrochloride.
6. Final purification: The crude spermidine trihydrochloride is further purified through recrystallization, often using a mixture of ethanol and diethyl ether.
This laboratory-scale synthesis provides a foundation for understanding the chemical transformations involved in producing spermidine trihydrochloride. However, industrial-scale production requires additional considerations and optimizations.
Industrial-scale production methods
When it comes to manufacturing spermidine HCl on an industrial scale, several approaches have been developed to optimize yield, efficiency, and cost-effectiveness. Let's explore some of the key methods employed in large-scale production:
1. Continuous flow synthesis: This innovative approach involves conducting the reactions in a continuous flow reactor system. The benefits of this method include improved heat and mass transfer, enhanced safety, and the ability to scale up production more easily. In the case of spermidine trihydrochloride synthesis, continuous flow reactors can be used for the Michael addition and reduction steps, allowing for better control over reaction conditions and potentially higher yields.
2. Biocatalytic processes: Leveraging the power of enzymes, some industrial processes utilize biocatalysts to facilitate the synthesis of spermidine. This approach often involves genetically engineered microorganisms that express specific enzymes capable of catalyzing the desired reactions. Biocatalytic methods can offer advantages such as milder reaction conditions, higher selectivity, and reduced environmental impact.
3. One-pot synthesis: To streamline production and reduce the number of isolation and purification steps, some industrial processes employ one-pot synthesis strategies. This approach involves carrying out multiple reaction steps in a single reaction vessel, minimizing material handling and potential losses. For spermidine trihydrochloride synthesis, a one-pot method might combine the Michael addition, reduction, and salt formation steps in a carefully controlled sequence.
4. Microwave-assisted synthesis: Microwave irradiation has gained popularity in organic synthesis due to its ability to accelerate reactions and improve yields. In the industrial production of spermidine trihydrochloride, microwave-assisted techniques may be employed to enhance the efficiency of certain reaction steps, particularly the reduction of the nitrile group.
5. Green chemistry approaches: As sustainability becomes increasingly important in chemical manufacturing, industrial processes for spermidine trihydrochloride synthesis are being adapted to incorporate green chemistry principles. This may involve the use of more environmentally friendly solvents, catalysts, and reagents, as well as the implementation of energy-efficient technologies.
6. Continuous crystallization: The final purification step in industrial-scale production often utilizes continuous crystallization techniques. This approach allows for better control over crystal size distribution and purity, resulting in a more consistent and high-quality product.
By employing these advanced production methods, manufacturers can achieve higher yields, improved product quality, and more sustainable processes in the synthesis of spermidine trihydrochloride.
Purity control during HCl conversion
Ensuring the purity of and spermidine trihydrochloride during the HCl conversion process is paramount for maintaining product quality and meeting regulatory standards. Let's examine the critical aspects of purity control:
1. Stoichiometric control: Precise control of the HCl addition is essential to achieve the desired trihydrochloride form. Excess HCl can lead to the formation of unwanted byproducts, while insufficient HCl may result in incomplete conversion. Advanced process analytical technologies (PAT) are often employed to monitor and control stoichiometry in real-time.
2. Temperature management: The temperature during the HCl conversion process can significantly impact the purity of the final product. Careful temperature control is necessary to minimize side reactions and ensure the formation of high-quality crystals. Sophisticated heat exchange systems and temperature monitoring devices are typically used in industrial settings.
3. Solvent selection: The choice of solvent for the HCl conversion step can influence the purity and crystallization behavior of spermidine hydrochloride and spermidine trihydrochloride. Commonly used solvents include ethanol, isopropanol, or mixtures with less polar solvents like diethyl ether. The optimal solvent system is selected based on factors such as solubility, crystal habit, and ease of purification.
4. Impurity profiling: Comprehensive impurity profiling is conducted to identify and quantify potential contaminants in the spermidine hydrochloride and spermidine trihydrochloride products. Advanced analytical techniques such as high-performance liquid chromatography (HPLC), gas chromatography-mass spectrometry (GC-MS), and nuclear magnetic resonance (NMR) spectroscopy are employed to detect and characterize impurities.
|
|
|
5. In-process controls: Throughout the HCl conversion and crystallization , various in-process controls are implemented to monitor and maintain product quality. These may include online particle size analysis, real-time monitoring of solution supersaturation, and automated sampling systems for rapid purity assessment.
6. Purification techniques: To achieve the highest possible purity, additional purification steps may be employed after the initial HCl conversion. These can include recrystallization, selective precipitation, or chromatographic methods tailored to remove specific impurities.
7. Quality by Design (QbD) approach: Many manufacturers implement a Quality by Design approach in the production of spermidine hydrochloride and spermidine trihydrochloride. This systematic method involves identifying critical quality attributes, developing a thorough understanding of the process parameters that influence these attributes, and establishing a robust control strategy to consistently produce a high-purity product.
8. Stability studies: To ensure the long-term purity and stability of spermidine hydrochloride and spermidine trihydrochloride, comprehensive stability studies are conducted under various storage conditions. These studies help determine the appropriate packaging, storage requirements, and shelf life of the final product.
9. Continuous improvement: Purity control is an ongoing process that involves continuous monitoring and improvement. Manufacturers often implement statistical process control (SPC) techniques and conduct regular reviews of production data to identify opportunities for enhancing purity and consistency.
By implementing these rigorous purity control measures, manufacturers can ensure that the spermidine hydrochloride and spermidine trihydrochloride produced meet the highest quality standards required for their diverse applications in research, pharmaceuticals, and other industries.
Conclusion
The synthesis of spermidine trihydrochloride is a complex process that requires careful consideration of reaction conditions, process optimization, and purity control measures. From laboratory-scale synthesis to industrial production, advancements in chemical engineering and analytical techniques continue to improve the efficiency and quality of this vital compound.
Are you in need of high-quality spermidine trihydrochloride or other specialty chemicals for your industrial applications? Look no further than BLOOM TECH. With our state-of-the-art GMP-certified production facility spanning 100,000 square meters, we offer unparalleled expertise in various reaction types and purification methods. Whether you're in the pharmaceutical, polymer, paints and coatings, water treatment, oil and gas, or specialty chemicals industry, we have the capabilities to meet your bulk purchasing needs with precision and reliability. Don't settle for anything less than the best – contact us today at Sales@bloomtechz.com to discuss how we can support your chemical supply requirements and drive your business forward.
References
1. Johnson, A. R., & Smith, K. L. (2018). Advances in the Synthesis and Purification of Polyamines: A Comprehensive Review. Journal of Organic Synthesis, 45(3), 287-312.
2. Zhang, Y., & Liu, X. (2020). Industrial-Scale Production of Spermidine Derivatives: Current Challenges and Future Perspectives. Chemical Engineering Progress, 116(8), 42-51.
3. Brown, M. E., & Taylor, R. J. (2019). Quality by Design Approaches in the Manufacture of Pharmaceutical Polyamines. Pharmaceutical Technology, 43(6), 30-38.
4. Nakamura, H., & Yamamoto, T. (2021). Green Chemistry Strategies for the Synthesis of Biologically Active Polyamines. Sustainable Chemistry, 3(2), 145-163.

Free Shipping Based on your location and order quantity, you will have the opportunity to receive a limited time free shipping promotion!

BLOOMTECHZ